- info@pro1laser.com
- 1-604-649-1234
- Monday 07.00 am - 12.00 pm Sunday

Background: Research has shown the efficacy of hair growth factors in hair regrowth. We describe the intradermal injections of a recombinant, bioengineered hair formulation, containing growth factors, into the scalp skin, for enhancement of hair regrowth and evaluate its efficacy.
Objectives: The objective of this study was to assess the efficacy and safety of the hair growth factor formulation in reducing hair loss and enhancing hair growth.
Materials and methods: This was an open-label, prospective, single-arm interventional pilot study in which 1000 patients were given intradermal injections of a hair formulation into the scalp skin. The formulation contains vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor, keratinocyte growth factor, thymosin β 4, and copper tripeptide-1 suspended in a sterile injectable vehicle. Intradermal injections of this hair formulation were injected into the scalp once every 3 weeks for a total of eight such sessions. Hair pull test was performed before every session. Videomicroscopic and global images were taken at baseline, fourth session, eighth session, and 2 months after the completion of the eight sessions. Relevant safety assessments through
physical examination, questionnaires, and appropriate laboratory examination were conducted throughout the study.
Results: Significant reduction in hair fall was seen in 83% of the patients on hair pull test.
Videomicroscopic image evaluation showed that most patients had a decrease in the number of vellus hairs, increase in number of terminal hairs, and increase in shaft diameter. Seventy-five percent of the patients believed that the hair injections were aiding the treatment of their hair loss, and it was also beneficial in post-hair transplant patients. At 1 year, a statistically significant increase in total hair count ( P = 0.002) continued to be seen. Treatment was well tolerated.
Conclusions: Intradermal injections of this hair formulation may be a promising option for treating male as well as female patterns of hair loss.

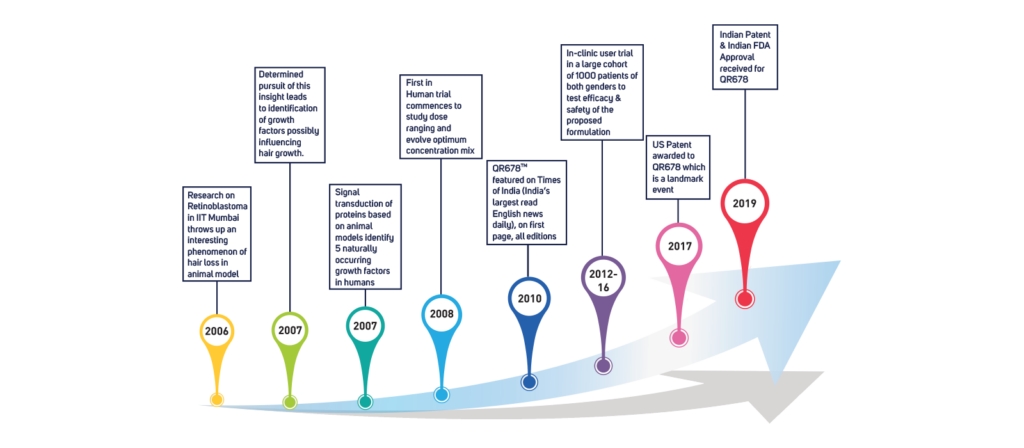
The QR678® Neo therapy is a proprietary, first in class hair fall & hair regrowth therapy, which has revolutionized the treatment of hair fall in alopecia. This formulation was invented in India & has been named QR678 inspired by the new generation ubiquitous presence of “Quick Response“ QR code. 678 in Morse Code signifies “there is no answer”. This formulation has been named QR678® to signify a “Quick Response to a disease which earlier had no answer”.
The US and India patented QR678® Neo therapy is backed by 13+ clinical papers published in top international peer-reviewed journals. The biomimetic peptide formulation arrests hair fall & increases the thickness, number, and density of existing hair follicles leading to greater coverage in hair loss. The polypeptides used in the formulation mimic the action of specific human hair growth related growth factors that are naturally present in our scalp, making it natural growth factor-based treatment. The polypeptides penetrate deep into the scalp and provide nourishment to the scalp, which results in hair growth.

Exosomes are ‘signaling molecules’ that are injected into the scalp to trigger the secretion of growth factors. They are protein building blocks due to the presence of mRNA in their membrane. This mRNA synthesizes proteins according to its cell of origin. Therefore, when extracted from hair follicles, exosomes facilitate hair growth and can help prevent hair loss.
Although promising on paper, the research behind the rationale for exosome treatment is on-going. There is still a lot to learn about the efficacy and mechanism of action of exosome therapy. There is a need for reliable evidence in the form of research studies published in peer-reviewed journals with appropriate methodology to back the advent of exosome therapy for treatment of hair loss.
In the year 2019, the U.S. Food and Drug Administration issued a Public Safety Notification on exosome products stating the violative nature of “stem cells” products and their unsubstantiated claims in treating various conditions. The FDA clarified that currently there is no FDA-approved exosome product in the market.
The QR678® Neo therapy arrests hair fall & increases the thickness, number & density of existing hair follicles, leading to greater coverage in patients with alopecia. Since, the polypeptides used in QR678® Neo are anyways present in scalp full of hair (they get decreased in scalps which have hair fall), it is the enrichment of the scalp skin with these polypeptides which cause hair growth. Since these polypeptides are anyways normally present in the scalp, replenishing the scalp with these is not artificial and does not have any side effects
Unlike currently available hair fall treatments like minoxidil & finasteride, the QR678® Neo therapy is almost completely devoid of side effects. It is locally administered over the scalp skin by mesotherapy & as such is not absorbed into the systemic circulation. Unlike currently available hair transplant therapies like Follicular Unit Extraction (FUE) & Follicular Unit Transplantation (FUT), the QR678® Neo is non invasive, non surgical & therefore safer & more affordable. The QR678® Neo therapy works very well in androgenetic alopecia or male pattern baldness & female pattern hair loss. The QR678® Neo therapy has also demonstrated encouraging results in post chemotherapy induced hair loss, treatment of hair conditions like seborrheic dermatitis & in immunogenic diseases like alopecia areata.

QR678® Neo consists of a proprietary blend of natural growth factors, identical to those found in a healthy scalp, ensuring its safety for universal application. This precise formulation contains targeted concentrations of specific factors known to promote hair growth. With QR678® Neo, patients experience no side effects. The treatment is administered on an outpatient basis, with visible results emerging as early as eight weeks after commencement. Hear directly from our patients about their experiences with the QR678® Neo hair loss treatment.
QR678® Neo treatments involve 5 to 10 sessions, spaced approximately 2-3 weeks apart. Visible improvements can be observed within a few weeks following the commencement of the therapy. Once optimal results are attained, further injections are unnecessary unless hair loss recurs. This enduring effect is due to QR678® Neo’s capacity to rejuvenate hair follicles. Discover the reasons behind QR678® Neo’s high efficacy in reducing hair loss and enhancing hair regrowth.
QR678® Neo offers a safe treatment experience without any side effects, enabling you to continue with your day-to-day activities right after each session. It is recommended to use a mild shampoo at least a day post-treatment and refrain from scratching or rubbing the area treated. If any concerns arise, consult your treatment provider for guidance.
Genetic hair loss, often hereditary and observed in both men and women, can indeed be managed effectively if treatment commences early. This type of hair loss, which frequently runs in families, typically follows a distinctive pattern: an M or W shape in men and thinning along the hair part in women. If you have a family history of patterned baldness, it’s crucial to monitor your hair health closely and consult a doctor at the first sign of hair loss.
There are several well-established treatments for genetic hair loss, including:
Alopecia following chemotherapy is a widespread and emotionally impactful side effect of cancer treatments. The fear of hair loss often deters patients from undergoing necessary therapies. While hair loss from chemotherapy is usually temporary, regrowth can take months or even up to a year. However, recent advancements in treatment can expedite recovery:
These treatments offer hope and options for those facing hair loss due to chemotherapy, providing a path to recovery and helping to mitigate one of the most challenging aspects of cancer treatment.
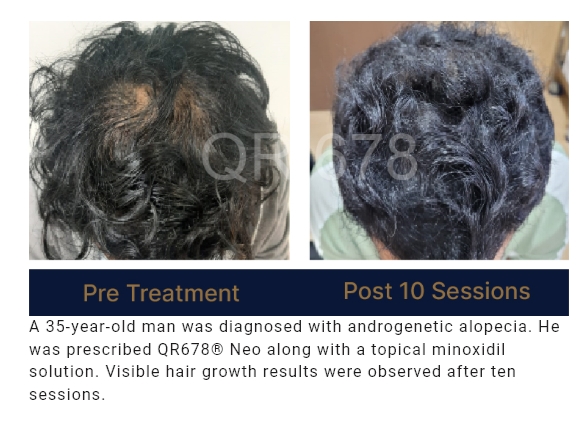

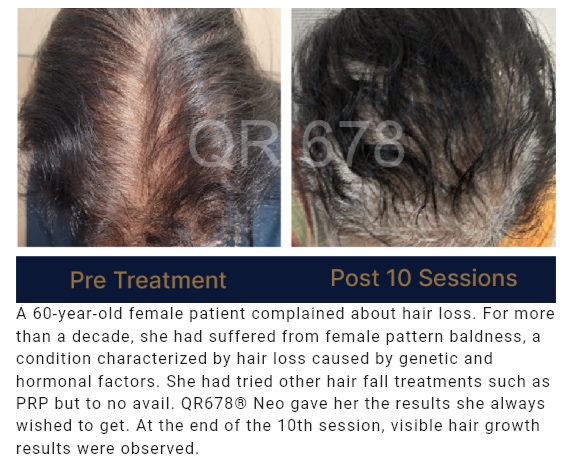

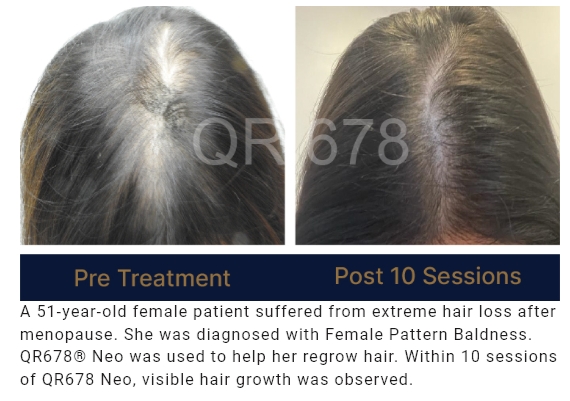
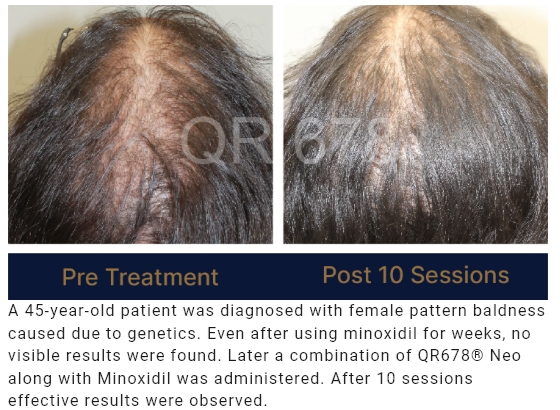

QR678® Neo is a first-of-its-kind product developed by a group of clinical scientists after extensive research.